About QI Determination
Sharing Our Work
A QI Determination must be received for all quality improvement work to be shared outside of the University of Chicago Medicine.
Quality improvement occurs on a continuous basis throughout the institution. Most quality improvement efforts are very local and will not be shared outside of the institution (e.g. Managing for Daily Improvement huddles). Quality improvement initiatives that are NOT shared outside of the institution are outside the scope of this policy.
Publication of a project has no bearing on whether it is human subjects research or quality improvement. You may wish to publish something if you believe others would be interested in learning about your activities without it being human subjects research. Conversely, some improvement projects are in fact better classified as human subjects research even when there is no intention of disseminating or publishing the findings.
A Formal Review Process
Defining Your Project
To protect our patients, investigators, and the institution, a formal review process is required to ensure clear distinction between human subjects research and quality improvement initiatives. QI Determination is required for all quality improvement projects that will be shared outside of UChicago Medicine. This process was implemented in 2015 in conjunction with UChicago Medicine leadership and the Office of Clinical Research (OCR).
Human subjects research, which aims to discover new generalizable knowledge, is governed by the Institutional Review Board (IRB) and other applicable policies.
Quality improvement projects, which are based on existing knowledge, do NOT incorporate human subjects research.
Regardless of whether an activity is quality improvement or human subjects research, teams must follow Institutional Security Policies and HIPAA regulations for protecting information, including ensuring that appropriate legal and business associates agreements are in place prior to sharing any individual or identifiable information outside of the institution (e.g. presentations at meetings, poster presentations, abstracts, and publications).
QI Determination Process
Meeting the Definition of QI
Please read; the QI Determination process has recently changed.
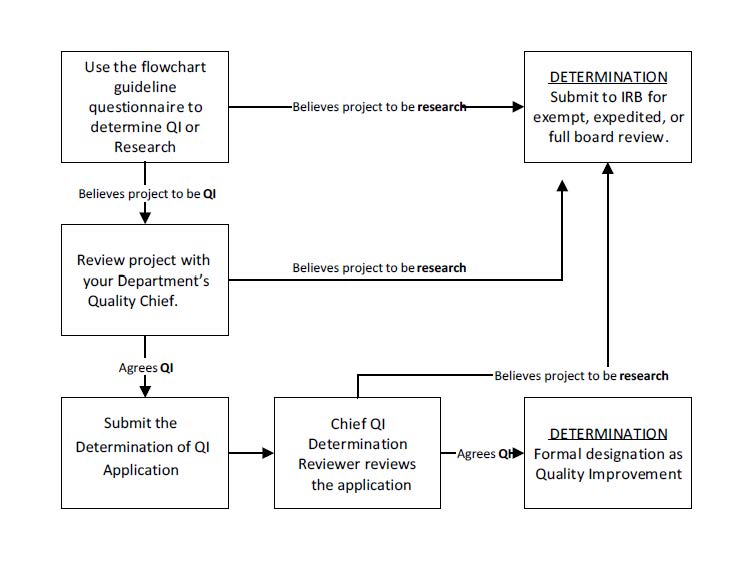
- Complete the online QI Determination form. There’s nothing more the submitter needs to do
- Department Quality Chief reviews submission
- Chief QI Determination Reviewer conducts second review of submission
- Submitter receives an email: either the submission meets the definition of QI, or the project is deemed research and should be submitted to the IRB for review
Additional Information
Definitions & Examples
- Human subjects research is governed by the IRB and other applicable policies.
- Human subjects research is defined in CFR §46.102 as:
- Research: a systematic investigation … designed to develop or contribute to generalizable knowledge
- Human subject: living individual about whom an investigator conducting research obtains
- Data through intervention or interaction with the individual, or
- Identifiable private information.
Quality improvement is sometimes defined in the following ways:
- “Quality improvement (QI) consists of systematic and continuous actions that lead to measurable improvement in health care services and the health status of targeted patient groups.”[1]
- Quality improvement is “the combined and unceasing efforts of everyone—healthcare professionals, patients and their families, researchers, payers, planners and educators—to make the changes that will lead to better patient outcomes (health), better system performance (care) and better professional development”[2]
- The pursuit of the triple aim: “Improving the U.S. health care system requires simultaneous pursuit of three aims: improving the experience of care, improving the health of populations, and reducing per capita costs of health care.”[3]
[1] U.S DEPARTMENT OF HEALTH AND HUMAN SERVICES, HEALTH RESOURCES AND SERVICES ADMINISTRATION. QUALITY IMPROVEMENT. 2011. ACCESSED 29 OCTOBER 2015 AT HTTPS://WWW.HRSA.GOV/QUALITY/TOOLBOX/508PDFS/QUALITYIMPROVEMENT.PDF.
[2] BATALDEN P, DAVIDOFF F. WHAT IS ‘‘QUALITY IMPROVEMENT’’ AND HOW CAN IT TRANSFORM HEALTHCARE? QUAL SAF HEALTH CARE 2007;16:2–3
[3] BERWICK D, NOLAN TW, WHITTINGTON J. THE TRIPLE AIM: CARE, HEALTH, AND COST. HEALTH AFFAIRS, 27, NO.3 (2008):759-769
Examples of Quality Improvement projects:
- A team changes the approach of scheduling in clinic and uses Lean principles to reduce waiting. To assess impact, they measure throughput, potentially preventable hospital admissions, and patient satisfaction with care, all of which are collected as part of routine operations.
- A guideline for managing pneumonia is published by a national specialty society. A multidisciplinary team adapts this guideline to UCM and implements it using PDSA tools. To assess improvement, the team uses data from the electronic health record on compliance with the guideline, patient outcomes and costs of care.
For more information regarding the background of quality improvement determination, read the following resources:
Contacts
Departmental Quality Chiefs
| Name | Department |
| Avery Tung, MD | Anesthesia & Critical Care |
| Anne Pohlman, APN Judie Holleman, APRN PPCNP-BC CPNP-AC CCRN |
Center for Advanced Practice (non-student) |
| Maggie Mueller, MD | Gynecology |
| Andy Davis, MD | Medicine |
| Ali Mansour, MD | Neurology |
| Nicole Bohr, PhD, RN Monica Gonzalez, MS, APRN, PCNS-BC, CCRN Students, please discuss with your preceptor before submitting a QI Determination form. |
Nursing (including DNP and MSN Students) |
| Sara Wallace, MD | Orthopaedic Surgery |
| Abbe Kordik, MD | Obstetrics |
| Peter Veldman, MD | Ophthalmology and Visual Science |
| Melissa Pessin, MD | Pathology |
| Allison Bartlett, MD | Pediatrics |
| Randall Knoebel, PharmD, BCOP | Pharmacy |
| Royce Lee, MD | Psychiatry |
| Stanley Liauw, MD | Radiation Oncology |
| Jonathan Chung, MD | Radiology |
| Vivek Prachand, MD | Surgery |
Requesting Data
Neither QI Determination nor IRB process guarantees resources. All data requests go through ACRES.
- Projects that will be shared go through QI Determination and then Privacy Office before ACRES request is granted
Resources:
- Data Analytics
- Data Sharing Policy
- Show Me the Data: learn more about the different types of data you can request
For more information, please contact the QI Determination Senior Project Manager, Kimisha Cassidy.
